
The reaction with chlorine and bromine are thermodynamically possible but they react very slowly forming corresponding hydrohalic and hypohalous acids. The reaction is spontaneous and strongly exothermic.ĢF 2(g) + 6H 2O(l) -> 4H 3O + (aq) + 4F¯ (aq) + O 2(g)
Fluorine is so strong oxidising agent that it oxidises water to dioxygen. The relative oxidising power of halogens can be further illustrated by their reactions with water. The overall energy released for these are less than those of F 2 and Cl 2. Since bromine occurs as liquid at room temperature, it involves enthalpy of fusion also, while iodine which is solid at room temperature, involves enthalpy of sublimation as well as enthalpy of fusion. The values of enthalpy changes of different steps are given below :

Thus, fluorine is very strong oxidising agent. As a result, AH overall is more negative for fluorine than for chlorine. The larger amount of energy released in step (iii) and lesser amount of energy required in step (i) overweighs the smaller energy released in step (ii) for fluorine. (ii) F 2 has very high enthalpy of hydration because of smaller size of the F¯ ion. (i) F 2 has low enthalpy of dissociation because of weak F-F bond. Flourine has less negative electron gain enthalpy, yet it is strongest oxidising agent because of the following reasons : The enthalpy change for this step is positive. The energy is needed to dissociate or convert molecular halogen into atomic halogen. This can be explained with the help of Born Haber cycle. The electrode potential of F 2 is maximum while that of I 2 is the minimum.This means that F 2 can be reduced most easily and I 2 is reduced least readily. This means that F 2 is the strongest oxidising agent while I 2 is the weakest oxidising agent. Chlorine has the highest negative electron gain enthalpy, so gaseous Cl atoms have maximum tendency to accept electrons and therefore, chlorine is expected to be strongest oxidising agent. However, chlorine is not strongest oxidising agent, but fluorine is the strongest oxidising agent. The decreasing oxidising power of the halogen as we go down the group is shown by their decreasing reduction potentials. In general, a halogen of lower atomic number will oxidize halide ion of higher atomic number and therefore, will liberate them from their salt solutions as given below : Fluorine is the strongest oxidising agent and oxidises other halide ions in solution or even in the solid phase. Therefore, it is most reactive among the halogens. Halogens have high electron acceptance property and therefore they have strong tendency to take up the electron :Īs a result, they act as powerful oxidising agents.

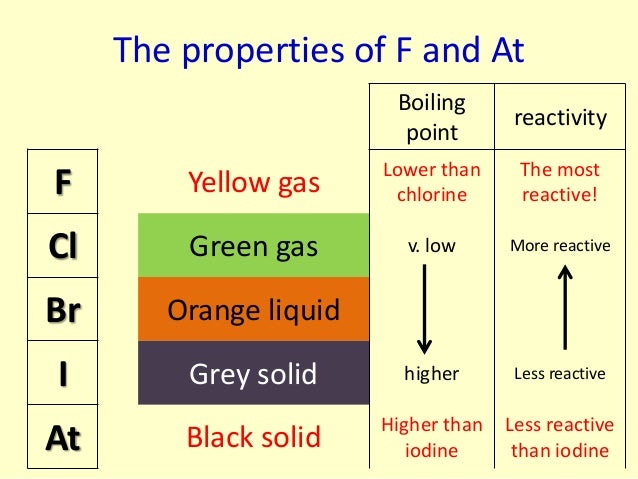
This is due to weak F-F bond because of the repulsion between the non-bonding electrons in the small molecule. Thus, halogens are very reactive elements due to their low dissociation enthalpies and high negative electron gain enthalpies. Fluorine has the lowest bond dissociation enthalpy. Halogens have very high negative electron gain enthalpy values and therefore, have very strong tendency to gain an electron. As a result, they can readily dissociate into atoms and react with other substances. The high reactivity of halogens is due to the following reasons :Īll the halogens have very low dissociation enthalpies. The reactivity of the halogens decreases down the group. Fluorine is the most reactive of all the halogens. The halogens react readily with metals and non-metals to form halides. 1.4 (4) Reactivity of Halogens towards Halogens.


 0 kommentar(er)
0 kommentar(er)
